Precision preparation
Why mRNA vaccines are so effective, and how they could help us prepare for the next influenza pandemic (11 minute read)
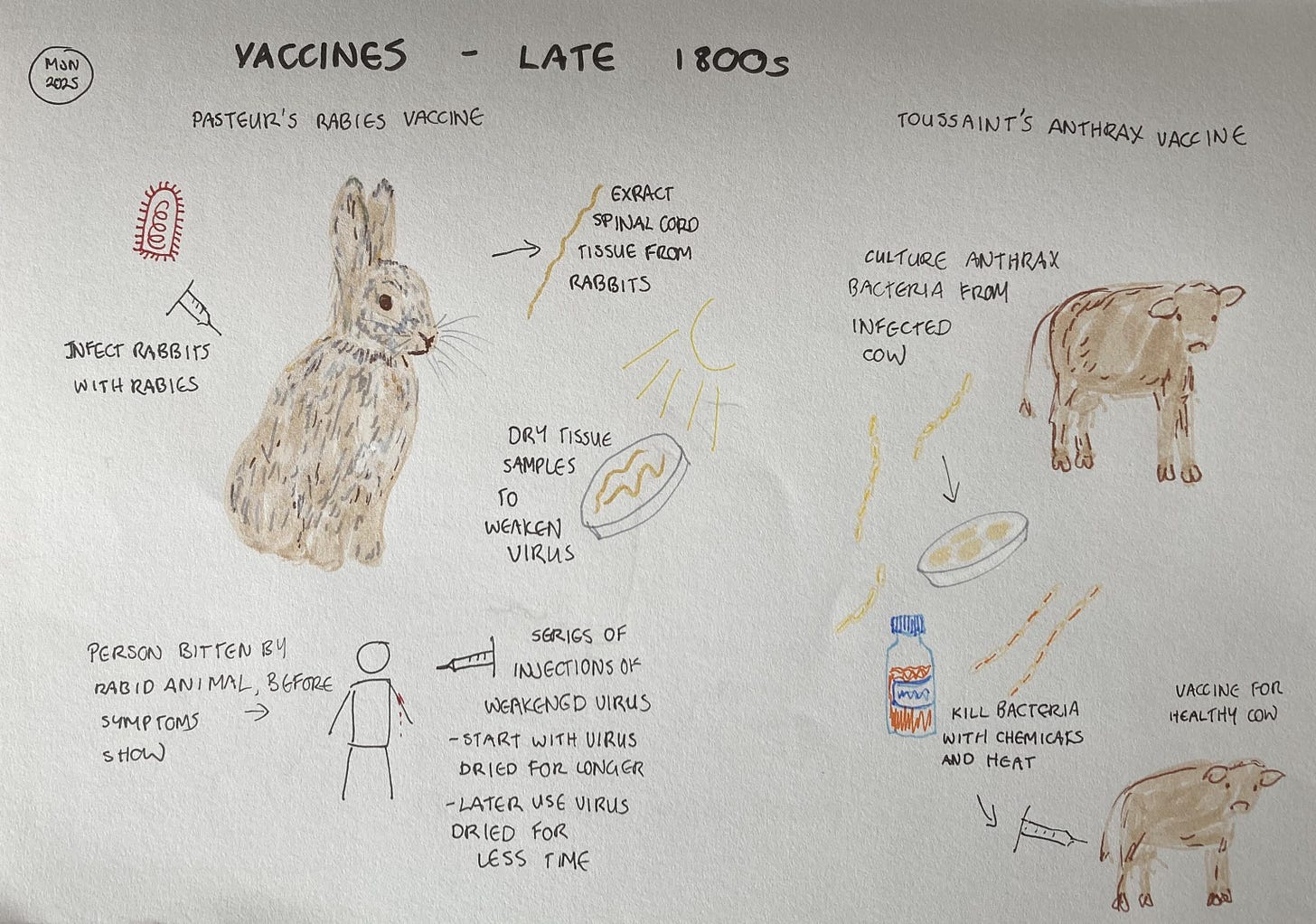
When I first learned about vaccine development, Louis Pasteur was presented as a singular genius who developed his rabies vaccine despite the scepticism of his peers. But history is usually more nuanced than it first appears, and so it is with Pasteur and his vaccines. He was no renegade, but a highly-regarded scientist. Nor was he the only one working on vaccines.
Before developing the rabies vaccines, Pasteur worked on livestock diseases, most notably anthrax. Out of this research came the idea of weakening a dangerous microbe and using that to induce immunity without causing disease. His idea is all the more extraordinary when you consider that it was the early 1880s. The idea of microbes causing disease was new, and the first accurate explanation describing part of the immune system wouldn’t be published until the end of the decade. To this day, we still use vaccines made using Pasteur’s approach.
Pasteur, however, had a blind spot. He believed that the weakened microbe was using up some essential nutrient in the body which would then not be available when the vaccinated animal or person was exposed to the disease. As a result, he was convinced that the microbe must be alive for a vaccine to work. When Henry Toussaint, at the Veterinary School of Toulouse, created a ‘dead’ vaccine against anthrax in cattle, Pasteur dismissed evidence that it worked and subsequently promoted his own, live vaccine.
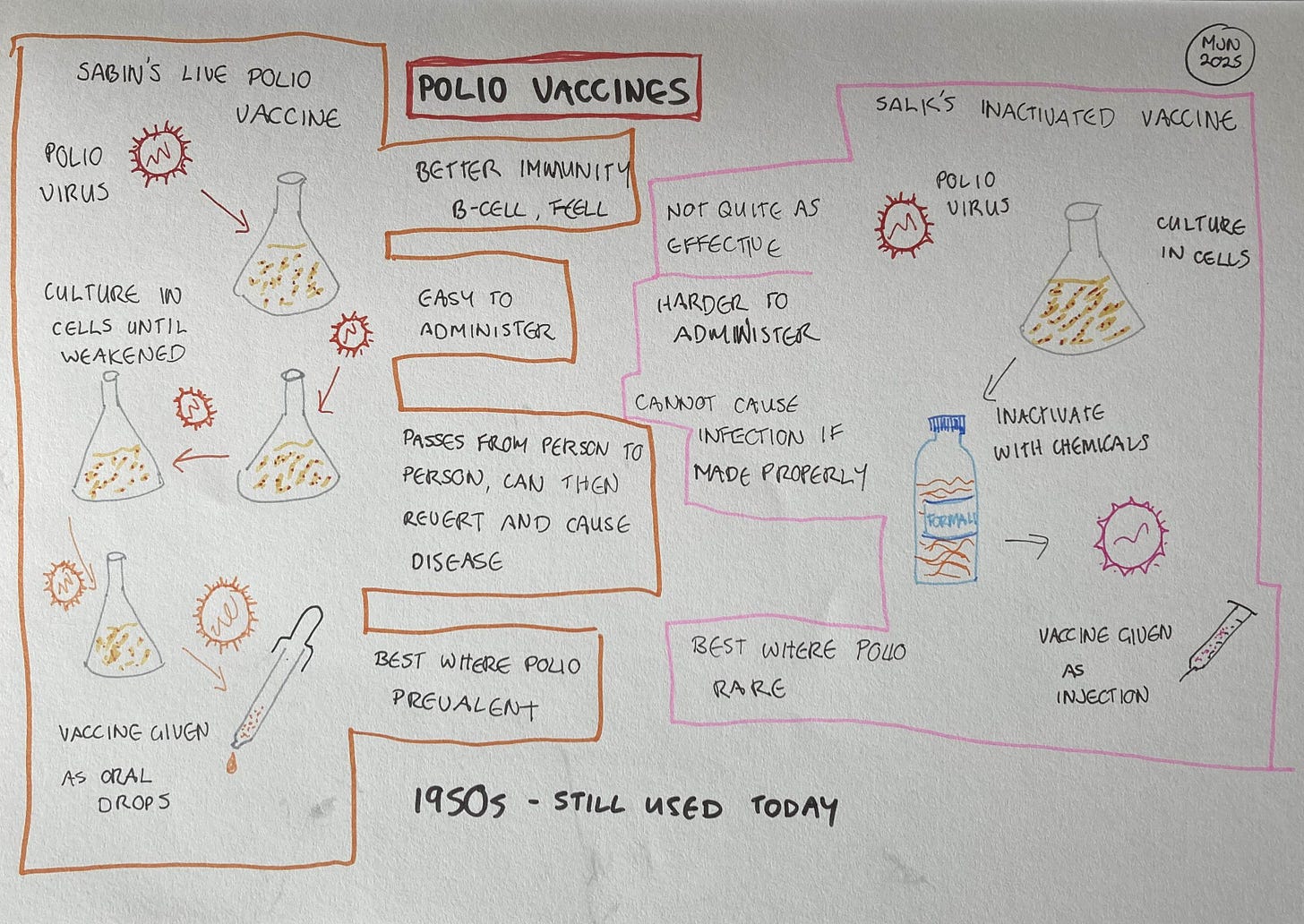
The relative merits of live and dead (or, for viruses, inactivated) vaccines, continued to be debated for many years. The debate perhaps reached a peak during the development of vaccines for polio. The bitter personal rivalry between the developer of the live vaccine, Albert Sabin, and the developer of the inactivated vaccine, Jonas Salk, contributed to the idea that one approach must be superior to the other. That perception was further reinforced by a deadly manufacturing problem at one of the facilities making Salk’s inactivated vaccine. Some batches weren’t fully inactivated, leading to thousands of infections and leaving around 200 children paralysed and 10 dead. Although it wasn’t a fault with the vaccine itself, the disaster contributed to the predominance of Sabin’s live vaccine for the next few decades.
Once again, however, there’s nuance to the question of which type of vaccine is better. This is one of the reasons I decided to speak with someone working directly with vaccines, so contacted the Malaghan Institute and spoke with Lisa Connor. I was interested to understand more about the relative merits of different vaccines, and to see where mRNA vaccines fitted into that picture.
Lisa explained that the polio vaccines give a good illustration of the differences, but they are specific to those two vaccines. “If you look at the difference between the live polio vaccine versus the inactivated vaccine, they're delivered in very different ways. One is injected, that's the inactivated version. It's really good at developing systemic immune responses, you're getting high antibody levels in the blood, which is important. But it isn’t getting to the mucosal surface of the gut, which is where the virus is entering. You have to have a live vaccine to survive that gut environment and to get an immune response to happen that way. So the live one is the most effective vaccine, if you're comparing the two, and it’s easier to deliver. They suggest the oral vaccine for areas where the virus is present. But it’s not as safe, because you can also get reversion to a disease-causing form of the virus from the live vaccine.”
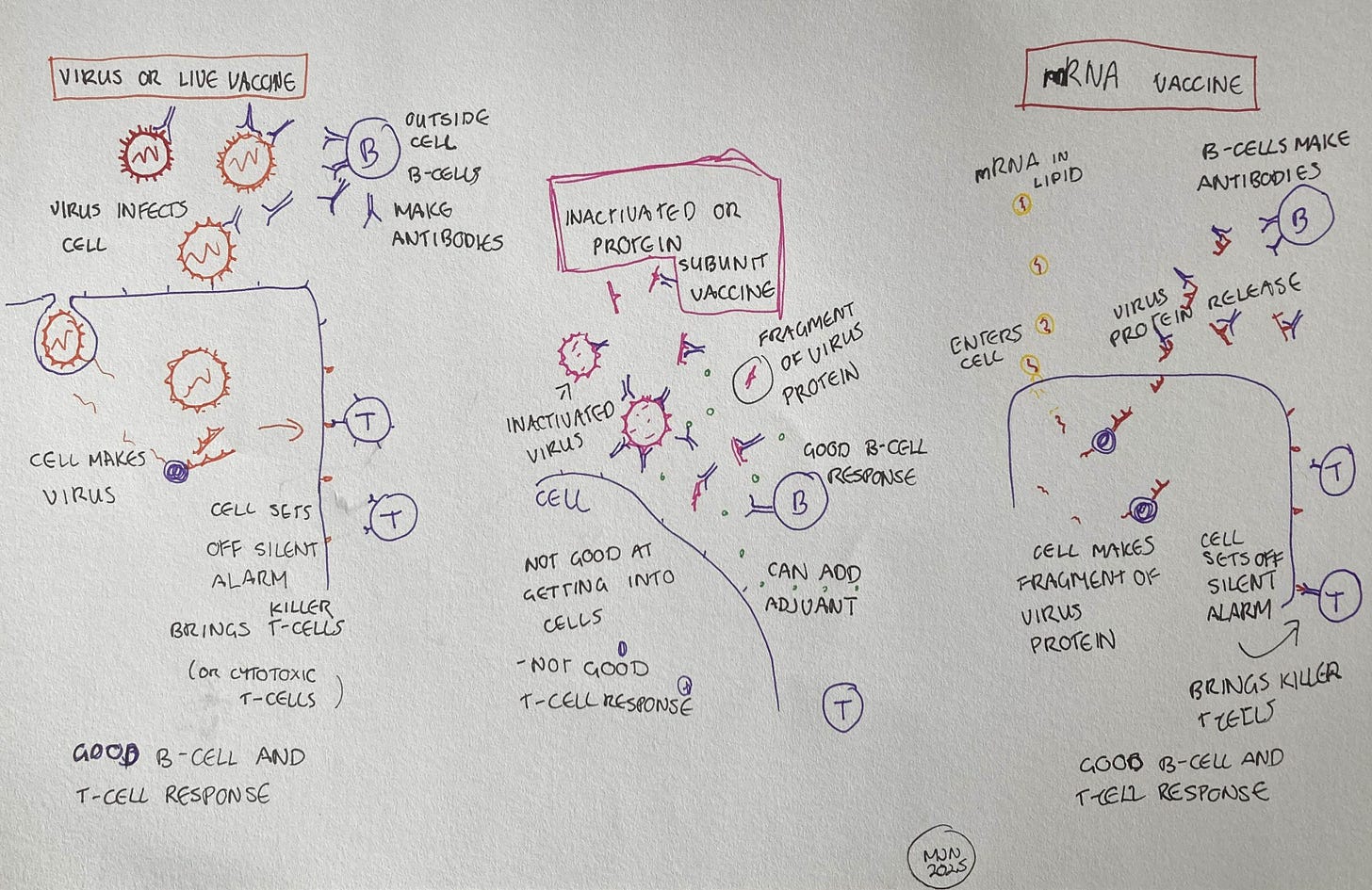
More generally, there’s an important difference in the way that live and inactivated vaccines work against viruses. Both kinds of vaccine trigger a response from our B-cells, which produce antibodies. These antibodies stick to the outside of the virus, and if they stick to the right spot on the virus, they can prevent the virus entering our cells. However, live vaccines are much better at triggering a response from our T-cells, because T-cells are activated by signals our cells send out when they are hijacked by a virus. A live vaccine contains virus capable of hijacking our cells, while an inactivated vaccine does not.
On the other hand, it’s the B-cell response, the antibodies, which prevent the virus from entering. In inactivated vaccines, this B-cell response can be enhanced. “You can add an adjuvant, another component, into the vaccine. The proteins of the virus will bind to it, and it forms a clump that’s ideal for B-cell responses, and they're the ones that make antibodies. So it's perfect for antibody responses.”
Both live and inactivated vaccines have been widely used and can be very effective, but the processes for making them were developed in the late 19th and early 20th centuries. “How live vaccines were made, back in the day, was through culturing the virus in cells in the lab, so it had no immune pressure. Because it didn’t have this pressure, it lost the elements which made it virulent. But this was quite an uncontrolled way of making vaccines. They didn’t understand the genetics of what was going on.”
The process for creating inactivated vaccines was also imprecise, Lisa tells me. “If the process uses formalin, like the inactivated polio vaccine, that can slightly alter the protein structure of the virus, compared to what the protein is in the original virus. So you're not always getting the right antibody response.”
In fact, it was just such a situation with a vaccine trial which inspired Lisa to make vaccines her career. “I heard a guest lecturer at the university talk about RSV vaccines. RSV is really important in New Zealand, because our babies have some of the highest infection and hospitalization rates in the world. Before her lecture, I was thinking that vaccines are always safe and they just do their job, but she told us about a clinical trial which was done in the 1960s where they induced an immune response which meant that when the baby was infected with RSV it had a worse outcome. The vaccine effectively enhanced the virus's ability to invade our cells and cause infection. That's when I became really interested in understanding more about vaccines and what all the nuances are. Why you can't just use one kind of vaccine which is going to cover everything, why some vaccines work really well and some don’t, why some diseases are a lot easier to develop vaccines for than others.”
Since the traditional approaches to making vaccines were developed, we have significantly improved our knowledge of viruses, genetics and the intricacies of the immune system. We’ve used this knowledge to make better, safer vaccines. An important advance in vaccine technology came with the development of protein subunit vaccines in the 1980s. Instead of using whole inactivated viruses, these vaccines contain just the parts of the virus’s protein shell which allow it to enter cells. The vaccine never actually enters the cell, since it lacks the other parts of the virus, but it induces the body to make antibodies against those proteins. “When we're looking at the efficacy of a vaccine, what we're looking for is high enough levels of those antibodies circulating that would be able to do that job. It’s not just having the antibodies present, but it's also having enough of them.”
Lisa has direct experience with protein subunit vaccines (also called recombinant protein vaccines), as her lab worked to develop the Kiwivax COVID-19 vaccine. “While the early part of the COVID-19 pandemic was a scary time, it was also an amazing time for science, because people just banded together. In New Zealand, we got the chemists, the protein structural biologists, the virologists and the immunologists into a room and talked about where our expertise lay and what kind of vaccine we could make in New Zealand. At the time, we didn’t have the ability to do it here, and we really had to rely on outside sources. So we looked at what it would take to make a vaccine in New Zealand.
“In 18 months, we had a product which gets a great immune response and is cross-reactive to the different variants. We learned a lot about the process of getting it from the bench to the bedside. We’re at the stage where we have a specific laboratory that has all the appropriate approvals to be able to make a product that can go into humans. They are currently going through the process with MedSafe to ensure that their labs are up to standard, so that they can make this product, and it's safe for New Zealand. Those thresholds for safety are extremely high, because we haven't had a vaccine for humans being made in New Zealand for a very long time.”
This is an important point. New Zealand has benefitted from research and development in countries with well-funded government research agencies and universities, as well as pharmaceutical industries, such as the USA. However, depending on the USA to develop new vaccines doesn’t look like a good idea right now. This is particularly the case with messenger RNA, or mRNA, vaccines, a powerful new technology which is currently being defunded in the USA.
What are the advantages of mRNA vaccines? Why should we develop our capability to make them?
The first advantage is that they are precise (a trait they share with protein subunit vaccines). “Certain types of antibody signal to cells called macrophages to come and engulf whatever the antibody is attached to. They do that so they can destroy the virus, but some viruses, such as dengue, use this as an easy way to get inside the macrophages and hijack them. So, these antibodies can enable the virus to infect the host even more efficiently. If you have that kind of virus, you don't want those types of antibodies in your vaccine, because you'll actually enhance the disease. So you have to understand all the nuances about the different pathogens.
“With mRNA vaccines, we can narrow down and specifically tell the immune system, I only want you to make a response to this part of the pathogen.”
The second advantage is that they have some of the benefits of a live virus vaccine, without the risks of reversion and growing large amounts of a potentially dangerous virus. This advantage is something which took me a while to understand, and it’s one of the reasons I went into detail about the virus infection process last week. Here’s a quick recap. When a cell is working normally, the cell uses its DNA as a template for making mRNA and uses that mRNA to make protein. When a virus, including a virus from a live virus vaccine, infects a cell, it hijacks the cell so that it starts making virus mRNA, and therefore virus protein instead. However, the hijacked cell also sends an alert to the immune system, triggering a response from a specific type of T-cell. It’s a little like a bank teller who hands over the cash to a bank robber, but also sets off a silent alarm.
mRNA vaccines induce the cell to make virus protein, just as a live virus would. Crucially, this triggers the same alert to the immune system, the silent alarm which tells the body the cell has been hijacked. Lisa tells me that “proteins are not good at getting into cells and triggering this response. But with mRNA, we are talking a completely different ballgame when it comes to activating this population of T-cells.”
The third advantage is speed. Lisa explains: “We made our COVID-19 vaccine using recombinant protein and an adjuvant, and it took us 18 months. We're a small group, but we worked tirelessly on this, around the clock. It was hard work. And it still took 18 months. There’s a lot of waiting. We would design the protein, and then we'd put it in the cells which make it, and then we had to purify it. You have all these hurdles that you have to get over. So it took a long time.
“But when mRNA technology came along, we looked at our protein vaccine, and wondered if we can make it into an mRNA vaccine. We made it within two weeks. It was ridiculous. When new variants would come along, it was just so quick to update the vaccine. Then we realised we needed to get more clever, because we don't want to be chasing these variants all the time. We want to make a vaccine that can recognize all of them. So we pulled together different design ideas and tested them. If we were working with proteins, we'd still be trying to look for that product years down the track, and we found it within 6 months. We can test them in cells and pinpoint really quickly which ones are working, we’re not using lots of animals, and we’ve just shortened the time. That’s what the mRNA platform can do.”
The Vaccine Alliance Aotearoa New Zealand and RNA platform are still working on getting the protein subunit vaccine approved, because doing that will test the system with a well established vaccine technology. But they are also looking at the mRNA vaccines we might need in the future. “We know there will be another influenza pandemic. History has told us that this will happen. The challenge is that the influenza virus is always changing, but there are some parts that never change, because those parts are really integral to the function of the virus. But we generally don't make immune responses to them. We’ve been figuring out how to use artificial intelligence and mRNA to make vaccines which will work against those parts, so they will work with different strains of the influenza virus. It's kind of preempting what the virus might be, a pre-pandemic vaccine. If we stockpile some of this for our frontline workers and vulnerable populations, we could reduce mortality and hospitalizations, and keep things at bay until a more matched vaccine is made.
“With this technology, we could actually be prepared this time.”