In early spring, I always wait for my trillium to push its leaves above the soil. It’s a quirky little plant, slow-growing in the best of conditions and easily swamped by the rampant weeds in my garden. I love any unusual plant, but this one is special for another reason. It was given to me, many years ago, by a friend of mine, Neroli O’Brien.
When I worked at the Allan Herbarium, she was a volunteer there, sitting at the desk opposite mine every Tuesday. Since the work was more manual than mental, we could talk all day with no loss of productivity, and so we did. She was bright and energetic, so I was stunned when one Tuesday she didn’t come in, and I found out that she’d become suddenly unwell over the weekend and had been diagnosed with terminal cancer. It had reached her liver, and she had just weeks to live.
Neroli faced her death with a combination of grace and pragmatism. Among the things she did was plan her own funeral, and she had a request for me – would I sing? So I learned, as requested, Panis Angelicus, by Cesar Franck, which had been sung at her wedding, and Climb Every Mountain from The Sound of Music. At her funeral, two months after her diagnosis, I held myself together until I’d sung for her – although I nearly didn’t when I saw her tramping boots on her coffin. She was only sixty-four.
Cancer touches everyone, and everyone has cancer stories – if not their own, then stories about friends and family. In 2022, nearly 29,000 people were diagnosed with cancer in New Zealand. Worldwide, there were around 20 million new cases. Because the progression of the disease and the treatments can be slow, many more than that are living with it at any one time. While cancer death rates are declining, it remains a leading cause of death – in 2019, around 17% of deaths around the world were attributed to cancer1.
These bare numbers say nothing of the immense suffering that cancer brings – the brutal treatments, the years of uncertainty, the loss of so many people far too young. So, when I heard that gene technology could be used to treat cancer, I knew it was an important topic I wanted to write about. However, it also seemed extremely complicated, so I decided to contact the Malaghan Institute, based here in Wellington, which is running New Zealand’s first clinical trial on a gene therapy treatment for a cancer called B-cell lymphoma.
I spoke with Dr Rachel Perret – who I learned read one of the same books as me when she was a child: “My favourite book was one about Louis Pasteur and the invention of the rabies vaccine. Louis Pasteur was beavering away in his lab in Paris, and everyone thought he was crazy to think he could protect people from disease by injecting something into the blood, but he kept on working. In the book, rabies was this nasty mob of little monsters in the body, and the vaccine was an army of tiny soldiers that marched through the syringe into the arm and fought the rabies and won. The story with the evil little monsters and the good soldiers really captured my imagination.”
I mostly remember the book frightening me, and that I needed to be reassured that rabies didn’t occur in New Zealand, but it inspired Rachel. “I thought that maybe I wanted to be a medical doctor, a paediatric oncologist, to be precise. But in my pre-med year at Otago University I had the most enthusiastic and exciting immunology lecturers. I realised that designing or inventing the cures for diseases would fit my personality and my interests better.”
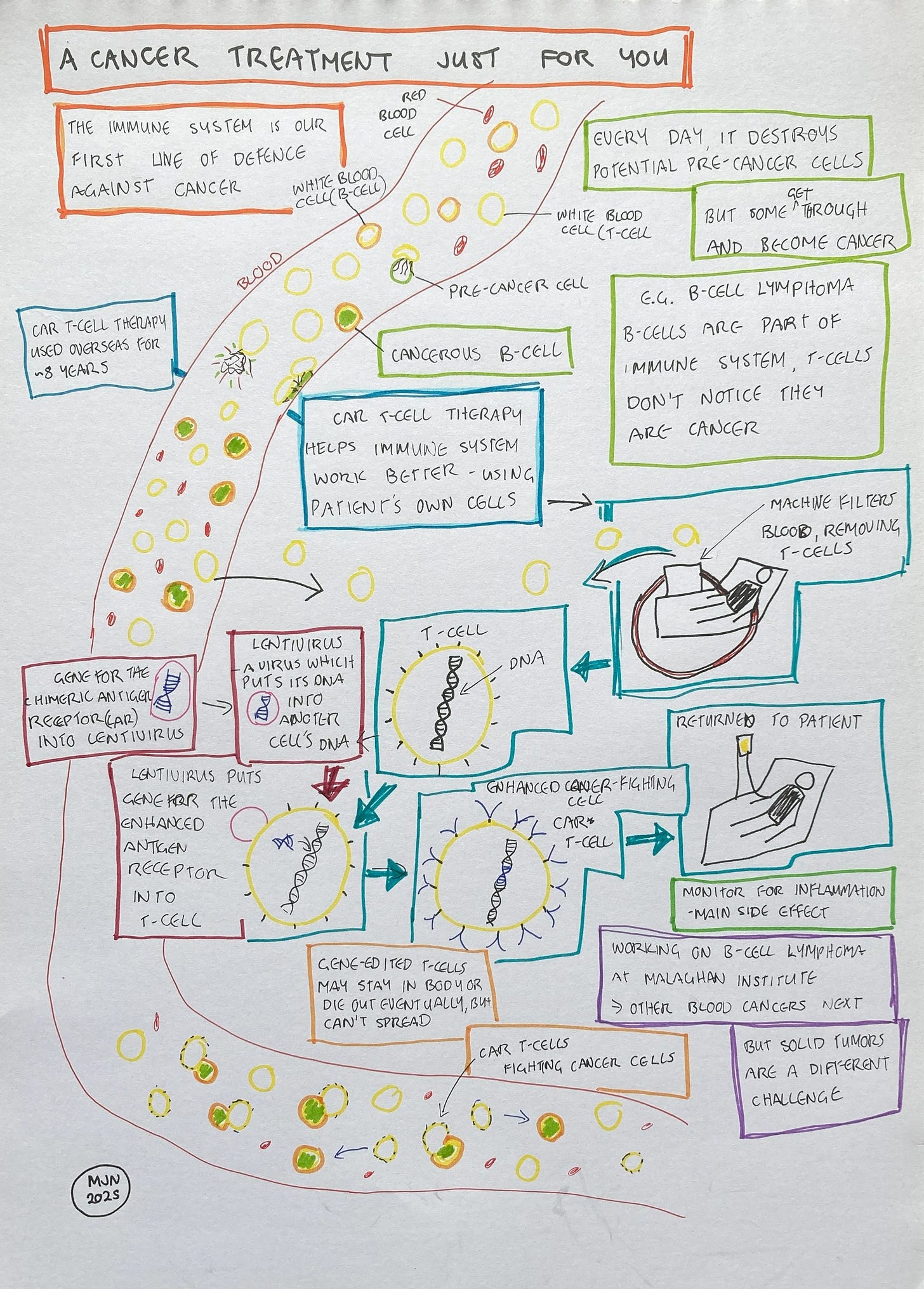
The treatment which Rachel is now working on is a kind of immunotherapy, which is quite different from the existing treatments for cancer, such as surgery, radiation or chemotherapy. It’s not aimed at the cancer directly, but helps the immune system so that it can fight the cancer more effectively.
Until Rachel explained it to me, I’d never realised that our immune system could target cancer at all. But, in fact, it’s extremely effective. “You're actually developing potentially pre-cancerous mutations at a rate of several a day, or maybe several dozen a day, depending on the conditions you live in. But most of the time your DNA repair systems and your immune system catch all those mutations and fix them, or delete the cells that were about to become cancer before they even have a chance. It’s the very rare ones that sneak through because they are too well hidden from the immune system, or too similar to the normal cells, that become full-blown cancers.”
The cancer that they’re treating at the Malaghan Institute is a particularly sneaky one. It’s a cancer of the immune system itself, affecting a kind of cell known as a B-cell. Rachel explains: “the cells that are cancerous look so similar to the normal, healthy cells that the T-cells [which are responsible for recognising harmful cells] are not even noticing it. It's not necessarily that they're not fast enough or need a bit of help. They can be completely blind to what's going on.”
To help the immune system recognise the cancerous B-cells, Rachel and her colleagues are using a kind of gene therapy, specifically a technique called CAR T-cell therapy. They are building on several decades of research and clinical trials. Following successful trials, the first CAR T-cell therapy was approved by the US Food and Drug Administration in 2017. It treated a kind of leukaemia which most often affects young children, and was used after other treatments had failed.
CAR T-cell therapy uses gene editing to make T-cells more effective and able to fight cancer, but it’s not simply a case of creating an improved human T-cell and then giving it to patients. If you or I donated our T-cells to someone with cancer, it just wouldn’t work. Our immune system recognises human cells which are not our own as foreign, and attacks them, which is why organ transplants are complicated. Instead, CAR T-cell therapy takes blood from a person who needs the treatment, extracts the T-cells, uses gene editing to reprogram the cells, multiplies the cells to create millions of copies, then puts the cells back into the patient. The cells that a patient receives during CAR T-cell therapy are their own, as far as the immune system is concerned.
What do the edited genes in these T-cells do? The genes contain the code for a protein which sits on the surface of the T-cell and is responsible for the T-cell recognising its target. The protein is known as a receptor, which accounts for the “R” in CAR, while the target is known as an antigen, accounting for the “A”. Rachel explains that they’ve taken bits of the immune system that they know work really well for recognizing cancers, and bits of the immune system that are really good at activating T cells to kill whatever they see. And they've fused those 2 things together to make a super powered receptor. Because the new antigen receptor contains parts which have been fused together, it’s described as chimeric, in reference to the ancient Greek myth about Chimera, a monster which was part lion, part goat and part dragon. All of this gives us a Chimeric Antigen Receptor, or CAR.
CAR T-cell therapy is a much more targeted treatment than traditional cancer treatments, such as chemotherapy. While the traditional cancer treatments have saved many lives, they can also have devastating side effects. Chemotherapy drugs affect cancer cells because they divide particularly fast. But, Rachel explains, “very important cells like your skin cells, your gut cells, and your immune cells all divide pretty fast, too, and that's why chemotherapy has so many bad side effects. Also, both chemotherapy and radiotherapy can lead to different cancers a few years later. CAR T-cells, being more specific, will have less of a risk of that.”
I also ask Rachel about the fate of the gene-edited T-cells, because one of the concerns people have about gene therapy is how it will affect other cells in their body. Another is whether they can pass the cells to other people or even the next generation. She explains that when someone receives these gene-edited T-cells, it doesn’t affect the DNA of any other cells in their body. Our CAR T-cells don’t pass on their DNA or turn into any other kind of cells. There is also no way someone can pass these cells on to anyone else, unless they donated their bone marrow for a transplant – something that someone who’d had gene therapy with a cancer of the immune system would not be doing anyway. There is no way for the edited genes to be passed on to the next generation either, because they don’t affect eggs or sperm.
This doesn’t mean that the treatment is without risks, however. “The risks that come from CAR T-cell therapy are not so much from the gene modification, but from the fact that we make T-cells very potent. The T-cells have lots of stimuli when they’re injected into the body, they get super activated and can become overstimulated.”
As part of their normal function, T-cells release chemicals known as cytokines, which are a signal to the rest of the immune system to cause inflammation. This is a normal process in the immune system, but Rachel explains that overstimulated T-cells can spit out lots of cytokines, leading to a general inflammation which can be harmful or even fatal. They can even cause inflammation in the brain, which she tells me is pretty scary.
However, these risks are now well-documented with CAR T-cell therapy. Rachel explains: “early clinical trials start with low doses of the CAR T-cells and monitor for symptoms very carefully. If we see signs of them, then we can maybe drop back the dose and make sure that we're in a safe spot. And there are treatments such as steroids which can reduce inflammation. What's actually pretty exciting about our New Zealand CAR T-cells, the ones in the clinical trial here, is that they have a very low toxicity profile compared to some of the ones being used overseas. And we've had zero cases of very severe inflammation due to the CAR T-cells so far, either in the blood or in the brain.”
The CAR T-cells which the Malaghan Institute are working on, in conjunction with colleagues in China2, are what’s known as 3rd generation CAR T-cells. “Our idea was to boost the signal of the CAR T-cells to make them stronger. We added an additional signalling part in the middle of the chimeric protein, and the new CAR T-cells were just as good at killing cancer cells in the laboratory, but they made a lot less of the inflammatory cytokines.”
So far, Rachel tells me that they’ve treated 30 patients in their phase 1 trial. “These were people who'd exhausted all existing therapies, they'd had a median of 4, or sometimes more, different types of therapy. That doesn't just mean one injection of chemo or radiation therapy. It might mean 6 months or a year on one kind of treatment and then moving to another kind. It was a hard task to ask the T-cells that we took out of their blood to become really good cancer fighting cells, because the patients' immune systems were already quite depleted. But because the phase 1 results were promising, in phase 2 we're now treating people who’ve only had one or two other kinds of therapy. So hopefully, their immune system will be in a better state, and they might respond even better.”
The current trial is looking at only one kind of cancer, but they have other cancers in their sights too. However, that doesn’t mean it will be easy. “This kind of therapy works pretty well on blood cancers, but not very well on solid cancers like lung cancer, breast cancer, pancreatic and prostate cancer. One of the big hurdles is that the cancer is a solid mass. That kind of creates a physical barrier around it. These cancers can also make things that we call suppressive factors, which tune down the immune system, or even kill immune cells. We're doing further research on how to overcome these barriers.”
I ask Rachel about the regulations, and how proposed reforms would affect her work. She explains that for clinical trials and eventual use of personalised/individual cell and gene therapies in patients, there is already a lot of regulatory oversight: ethical regulation (HDEC), gene therapy regulation (GTAC), medical regulation (MedSafe), and consultation with Māori through Te Whatu Ora Research Advisory Group - Māori (RAG-M). “For phase one clinical trials, it’s complicated to set up all the regulatory paperwork, and it's costly. It takes a long time to set everything up. And if you have to make any change, you have to start all over again from scratch. This can considerably delay access to potentially life-saving therapies”.
The treatments they develop will also need to be approved by Medsafe and funded by PHARMAC before they can be more widely used. She supports this kind of therapy being excluded from further regulation by the Environment Protection Authority, or a proposed new gene technology regulator, as they are already regulated by the strict rules for medicines. She points out that the rules on gene technology are aimed at managing risks to the community and wider environment, not personalised treatments that are injected directly into individual patients, which don't pose a risk to the wider environment, or to the public.
While the process to bring this treatment to New Zealand has been slow so far, Rachel explains that it may become faster. “We're thinking about the future. How can we move on from just this type of cancer? One idea is to develop what we call a phase 1 trial platform, where you'd have a series of clinical trials approved by regulators. The aim would be to assess new versions of CAR T-cell treatments, starting with the simplest version of a therapy administered at low doses. After a few patients, if they weren't getting any benefit but the treatment was safe, you could make small refinements that could boost the effect. Or the treatment could be assessed in one cancer type, then broadened to another cancer type if shown to be safe.” In fact, these treatments may have even wider uses. “These same CAR T-cells could also be used to treat autoimmune diseases that are caused by B-cells. And so that would actually allow us to help many more people.”
That’s a couple years away yet. But with the success of CAR T-cell therapy overseas, and the progress of the clinical trials here, it shouldn’t be too long before the first CAR T-cell therapy is approved in New Zealand.
In subsequent years, the proportion of deaths caused by cancer dropped, due to the arrival of COVID-19, which distorted the statistics.
The Malaghan Institute is working with Professor Li Peng at the Guangzhou Institutes Of Biomedicine and Health.





Thank you for a clear explanation of a complex topic.
Cancer is certainly a life-altering diagnosis.
Very interested to read that this process may have a place in dealing with autoimmune diseases.
This is a great read Melanie. Thank you.