Speed bumps
How was the Oxford/ AstraZeneca vaccine developed so quickly, and what does that have to do with genetic modification?
Plans to vaccinate the world against Covid-19 have had a setback in the last couple of weeks, with a number of countries suspending use of the Oxford/ AstraZeneca vaccine. This was not the first setback this vaccine encountered. Earlier, the French president Emmanual Macron had claimed the vaccine wasn’t effective in people over 65, and a number of European countries registered it for under 65 year old people only. Then there was the row between the manufacturer and the EU about supply of the vaccine, with the EU accusing AstraZeneca of supplying other countries at the expense of the EU. And, right back before any vaccines had been approved, the trial of this vaccine was suspended.
Given the speed of vaccine development, approval process and rollout, as well as the huge numbers of people being vaccinated, some setbacks were probably inevitable. But it’s a blow, because an effective vaccination programme needs the trust of the public, and stories like the ones I’ve mentioned above undermine that trust. It’s hard to know what to believe, and hard to shake the feeling that there must be something wrong. So what’s going on with this vaccine? Is there really a problem?
For New Zealand, questions about the Oxford/ AstraZeneca vaccine are academic, as New Zealand is doing all vaccinations with the Pfizer/ BioNTech vaccine. But the Oxford/ AstraZeneca vaccine has been approved in 81 countries, it’s being manufactured by the world’s largest supplier of vaccines, the Serum Institute of India, and it’s one of the main vaccines being delivered by COVAX, which is supplying vaccines to low and middle-income countries. It’s a critically important vaccine being given to many millions of people around the world. If there’s a problem it could cost many lives. But thousands are dying of Covid-19 every day. Any delay in vaccinating people who are at immediate risk will cost lives, without a doubt.
The most recent safety concerns about the vaccine came when a small number of recently vaccinated people in the UK suffered rare and dangerous blood clots, and one of them died. In Britain, where 11 million people have received at least one dose of the Oxford/ AstraZeneca vaccine, there were five cases. A further 13 cases were reported in Europe. Those numbers show just how difficult it is to be absolutely sure about the safety of any vaccine or medication. Clinical trials may include tens of thousands of people, but they can’t test for reactions that may occur in one out of every 2 million people.
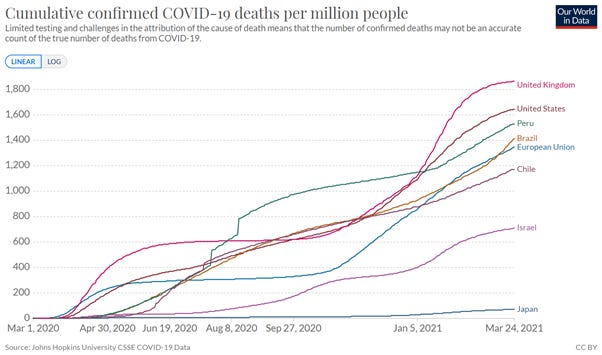
Even a rare side effect can be frightening, though. It’s horrible to imagine that you or someone you know might be the one person in 2 million. But it’s also important to keep the numbers in perspective. Right now, in the EU, more than 2500 people have died of Covid-19 for every 2 million in the population. In the United States, that figure is closer to 3300, and in Britain, it’s a truly awful 3500. Looking at those numbers, it may be easier to accept a blood clot risk of one in 2 million.
But that is assuming the blood clots were caused by the vaccine. And we can’t even be sure of that. After all, if you vaccinate 11 million people, chances are that some of them are going to develop health problems soon after being vaccinated, simply as a result of chance. Currently, medical regulators in Britain and Europe are saying that there is no proven link between the reported blood clots and the vaccine. It’s possible that the vaccine caused the clots, but it’s also possible that it didn’t.
The picture is similar for the cases of spinal cord inflammation, known as transverse myelitis, that occurred during the trial of the vaccine. There were three cases of transverse myelitis during the trial – two in people who had received the vaccine and one in a person who had received the placebo (for more information about the methods for clinical trials, see my previous article that looked at the Pfizer/ BioNTech vaccine). Because transverse myelitis is a serious condition – it can lead to permanent disability – the cases resulted in a pause of the trial. In the end, researchers concluded that one case was unlikely to be caused by the vaccine, as it occurred in a person who already had multiple sclerosis, and multiple sclerosis is a known cause of transverse myelitis. The other case, which occurred 14 days after the second shot, may have been caused by the vaccine, or it may not.
The idea that we can’t be sure about rare side effects is deeply dissatisfying. It also leaves medical regulators in an unenviable position. What’s the best thing to do when such cases come to light with an approved vaccine? Were the countries who suspended vaccination right? Or did they put lives at risk by halting their vaccine programmes in the middle of a deadly pandemic?
There’s no right answer to this question. Halting the vaccine programme means that people who would have been protected by the vaccine will catch the virus, and may even die. Continuing to vaccinate may end up costing lives if the vaccine is truly unsafe. Even if the vaccine itself is safe, there’s always the possibility of a bad batch. In the tragic case I wrote about previously, when measles vaccination in Samoa killed two babies, it was an error in preparing the vaccine that rendered the vaccine deadly.
There are other risks as well. Halting a vaccine programme because of safety concerns may undermine confidence in the vaccine and make people more reluctant to accept it. But not halting the programme could also undermine confidence, not only in the vaccine but in the whole regulatory system. We need to be able to trust that regulators won’t pretend everything is fine when it’s not.
But it isn’t just the possible side effects and debates about safety that have affected the Oxford/ AstraZeneca vaccine. There are also questions about how effective it actually is, especially in older people. The questions were not prompted by evidence that the vaccine didn’t work, but by issues with the data. Only 12% of participants in the phase III trials were over 55. This meant that when the first results of the trials were published in January of this year, there wasn’t enough evidence to draw a conclusion for this age group. There is more recent evidence that the vaccine does work for older age groups, but the words and actions of some politicians have sown needless uncertainty.
The controversies I’ve mentioned are the kinds that have occupied mainstream media, but the fringes of social media have seen others. Among the less plausible was a claim, reported and debunked by Reuters, that the Oxford/ AstraZeneca vaccine would genetically modify anyone who received it so that they’d no longer be classed as human and would require a “Covid slave passport” to move about. All of this, the claim explained, was part of a plot by wealthy Zionists. I wouldn’t have given the claim any attention if it didn’t allude to, but ultimately miss, an important point about the vaccine. Receiving the vaccine certainly does not make somebody a genetically modified organism. But the vaccine itself is a genetically modified organism, and that’s something that has had relatively little attention in the media.
The Oxford/ AstraZeneca vaccine works in a similar way to the Pfizer/ BioNTech vaccine, in that it tells human cells to produce the Covid-19 spike protein. I’ve explained this in more detail before, so I’ll just give a brief summary here. The vaccine contains a message – a piece of genetic material – that tells the cells to make the spike protein. When the cells make the protein, the body recognises it as something that doesn’t belong and makes antibodies. Those antibodies are then ready to help the body fight off the real virus if the person later catches it. The idea of a vaccine that tells human cells to make a virus protein does sound alarming, and it’s perhaps understandable that someone could think it means genetically modifying people. But telling human cells to make virus protein is what every virus does, every time someone is infected. I’ve previously explained this process when I talked about Covid-19 testing.
Where the Oxford/ AstraZeneca vaccine differs from the Pfizer/ BioNTech vaccine is in how the genetic message is delivered. In the Pfizer/ BioNTech vaccine, the message is carried in a molecule called messenger RNA. It’s relatively cheap and easy to manufacture compared to conventional vaccines, but it needs to be stored at extremely cold temperatures. In the Oxford/ AstraZeneca vaccine, that genetic message is carried inside another virus. It means that the vaccine is much easier to store – it can be kept at standard fridge temperatures. But it also means that it’s a genetically modified organism.
At a time when vaccine confidence is desperately needed, it may seem unwise to start creating new vaccines using potentially controversial technology. It means that we don’t just need to have confidence that the vaccine works and is safe – we also need to overcome our suspicions of genetic modification. But there’s one very compelling reason that this vaccine was one of the first to be approved – by the time Covid-19 hit, researchers at Oxford University had already created a very similar vaccine to the related Middle East Respiratory Syndrome virus, or MERS. That vaccine was moving through the stages of development and testing at the usual sedate pace, but when a vaccine for Covid-19 was needed, they were already halfway there.
The virus used to make the Oxford/ AstraZeneca Covid-19 vaccine, as well as the experimental MERS vaccine, is a type of common cold virus that normally infects chimpanzees, not people. Initially, researchers tried using human common cold viruses to produce vaccines, but these encountered various problems – not least that many people already had antibodies to them. The chimp virus rarely, if ever, infects humans, meaning that people won’t already have antibodies that might make the vaccine ineffective.
But taking a virus from chimpanzees, modifying it and then injecting it into people raises a lot of questions that aren’t answered by standard clinical trials for vaccine safety and effectiveness. Exactly what modifications have been made? Will the virus in the vaccine get into the environment? What if the virus mutates? How sure can we be that this technology is safe?
Because there are extra questions about their safety, genetically modified vaccines face extra hurdles when it comes to being approved. Not only must the vaccines be approved by medical regulators, but in some countries they need to be approved by the organisations responsible for managing genetically modified organisms. It means that we have a chance to look at extra information about these vaccines, and understand more about the risks. And that’s what I’ll be looking at in my next article.
Speed bumps: part two
Since I wrote my previous article on the Oxford/ AstraZeneca vaccine for Covid-19, reports of blood clots associated with the vaccine have continued to come in. Although they still say that the link is “possible”, the European Medicines Agency now reports
Do you know someone who might enjoy The Turnstone? Please forward it to them.
Did you receive The Turnstone from a friend? You can subscribe and receive it directly every week.
Do you want to see more of The Turnstone? All of the stories can be found in the archive, here.