Test drive
How can we make good decisions about gene drives for malaria control? (10 minute read)
A cascade of white, frothy netting falls from the ceiling, engulfing the double bed below. It’s the most spectacular mosquito net I’ve ever seen, a luxurious touch to the clean but otherwise average hotel room at a conference centre on the outskirts of Nairobi. The net has metres of fabric suspended from two curtain tracks, running from the head at each side of the bed and overlapping at the foot. The curtain track is enclosed in a pelmet and the fabric pools on the ground, so there’s no gap at the top or bottom. I’ve slept under mosquito nets many times, but never one like this.
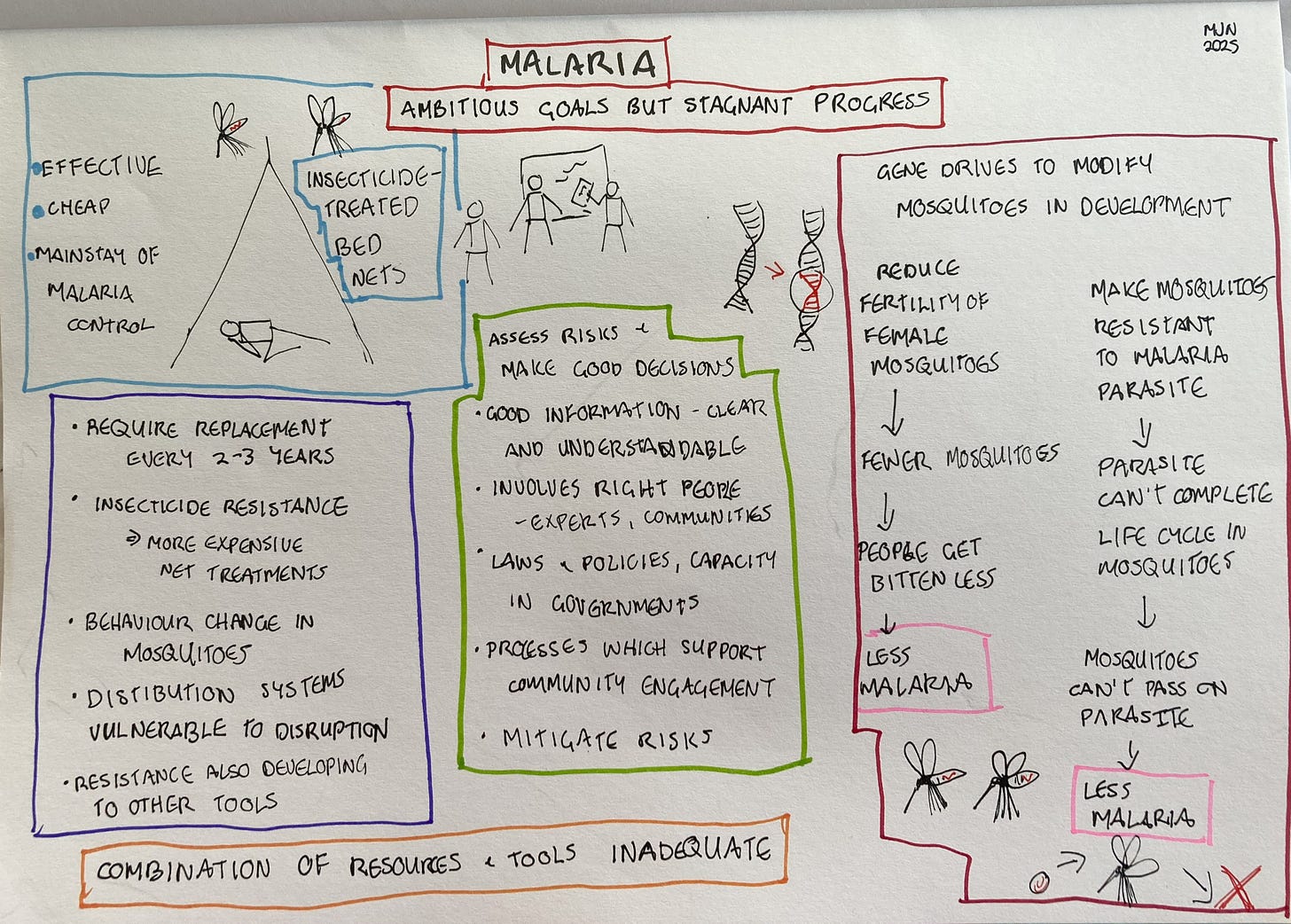
The mosquito net at my Nairobi hotel was mainly for comfort rather than safety, since there’s not much chance of catching malaria in Kenya’s capital. But for millions of people in Africa, mosquito nets are a matter of life and death. For decades, insecticide-treated nets have been the mainstay of malaria control, because sleeping under them prevents bites from the night-biting mosquitoes which transmit malaria parasites. From the year 2000 to 2015, nets prevented an estimated 450 million malaria cases. They were the single most important factor in the major reduction in malaria cases and deaths since 2000.
In 2015, a new strategy for malaria set the ambitious goal of a 90% reduction in malaria cases and deaths by 2030. Insecticide treated nets are central to that strategy. A number of countries, including Sri Lanka, El Salvador and China, have since eliminated malaria, and others, such as India, have achieved great reductions. However, some countries have gone in the opposite direction. As a result, the total number of malaria cases has actually increased since 2015, as has the number of deaths. Even when population growth is accounted for, the numbers are just holding steady, and certainly not decreasing at the rate needed to achieve the strategy’s goals.
Why has progress halted? Undoubtedly, conflicts such as those in Myanmar, Ethiopia and Sudan, as well as the worsening insurgency in the Sahel1 region, are part of the problem. So, too, is the disruption caused by the COVID-19 pandemic. But the problem is deeper than that.
In the leadup to World Malaria Day, on the 25th of April, I spoke with Dr Michael Santos, whose work at the GeneConvene Global Collaborative and the Foundation for the National Institutes of Health supports research on diseases which are closely linked to health inequities, such as malaria, tuberculosis and HIV. Mike explains that there’s no single reason that we are struggling to make progress with malaria. It’s that the combination of existing tools and resources is simply not sufficient to achieve the goals.
Insecticide-treated nets provide a good illustration of this problem. They are extremely effective, but they last only 2-3 years. Replacement nets need to be funded, and any disruptions to distribution will leave people vulnerable to malaria again. Mosquitoes have also become resistant to the insecticide used in many areas, so the World Health Organisation is now recommending nets treated with additional chemicals in these areas. But this comes at increased cost. In some areas, too, changes in mosquito behaviour have been reported, with more bites outside the times when people are usually sleeping under nets.
Of course, nets aren’t the only tool. There are a number of ways of preventing mosquito bites, as well as vaccines and treatments against the parasites. In theory, every one of the nearly 600,000 malaria deaths each year is preventable. But we don’t have unlimited resources, and in the real world, we are just holding the line. Mike tells me that the world needs both new resources and new tools, so that malaria control is both more effective and more sustainable.
This is where Mike’s work comes in. “The area that I am most focused on is leading a program called the GeneConvene Global Collaborative. This is an initiative that supports informed decision making about genetic biocontrol approaches, specifically for public health, like prevention of vector-borne diseases.”
Genetic biocontrol refers to any technique which involves modifying the genetic material of an organism in order to control a pest or disease. It doesn’t necessarily involve what we normally think of as genetic modification, though. The first kind of genetic biocontrol was developed in the 1950s and involves releasing large numbers of male insects which have been sterilised with radiation. When the sterilised males mate with normal females, the females lay infertile eggs, leading to reduced numbers of insects hatching in the next generation. This approach was used to eradicate a parasitic fly from North and Central America and parts of the Caribbean in the 1950s and has been used for other pests since then.
Gene technology has opened up new possibilities for genetic biocontrol. Mike explains: “There have been field trials of gene technologies in the mosquitoes that transmit viruses like dengue and yellow fever, and preliminary trials for those that transmit malaria. But these are what people call self-limiting, meaning that the genes that are introduced may persist for some number of generations, but they're not intended to persist indefinitely. Generally, they're decaying away.
“However, another form of gene technology is a gene drive, where a genetic change is introduced with an intent for it to persist in the population, and with an expectation that it would spread across interbreeding populations. There are many potential applications, but malaria is such a big and persistent challenge. And, unfortunately, a challenge, where progress has been stagnating. The magnitude of the current problem, with a child dying of malaria every minute, certainly provides a powerful motivation. That’s probably why these genetic approaches have advanced fastest in the case of malaria.”
Mike says that researchers are looking at two different ways of controlling malaria using gene drives. The first is aimed at suppressing the mosquito population. “It’s introducing a change that would reduce female fertility in the malaria vector mosquitoes. They’ve used computer models and have also tested it in large cage experiments and found this decreases the population of mosquitoes over time, and thus reduces biting and malaria transmission.
“The other approach involves interrupting the part of the malaria parasite life cycle that occurs within the mosquito. Malaria has a much more complicated life cycle than something like a virus. The life cycle stage that is acquired when a mosquito bites an infected person is not the same as the life cycle stage that is transmitted and infects another person. So, there's an opportunity to modify mosquitoes so that even if they bite someone who's infected, then acquire the parasite, the parasite wouldn't be able to complete its life cycle in the mosquito. So when somebody is bitten later on, they wouldn’t acquire malaria.
I asked Mike how far away gene drives are from being tested or used. “So far, no field trial of a gene drive in any species has occurred. Whether or not there are field trials depends on regulatory decisions made by countries. But both of these approaches are developed to a level where researchers would be ready to apply to regulators for field trials in the next two or three years.”
When researchers reach this point, good risk assessment and regulation becomes crucial, and that’s where the GeneConvene Global Collaborative has its focus. Mike explains: “Our role is to support informed decision-making, both by the researchers that are involved in developing these technologies, but also particularly on the governance side. We are working with partners like the African Union Development Agency to strengthen regulatory capacity in malaria-endemic countries where some of this research is going on right now. GeneConvene has team members that were risk assessors in the US Food and Drug Administration and the US Department of Agriculture in previous roles.”
The regulation of gene technologies, including gene drives, is governed by an international agreement known as the Cartagena Protocol on Biosafety, which is part of the Convention on Biological Diversity. Included in the Cartagena Protocol is a section which outlines the risk assessment approach for what are termed living modified organisms, meaning those which are developed using modern gene technology. Most countries have signed the Cartagena Protocol and use it as the basis of their systems for regulating gene technology.
Mike tells me: “There's a lot of interest in gene drives and their risk assessment among parties to the Convention on Biological Diversity. At their most recent meeting, one of the topics was risk assessment guidance specifically for gene drives for mosquitoes. That guidance was developed through a technical expert group, and one of GeneConvene’s team members was one of the experts. There's now fairly extensive additional guidance on how you might apply risk assessment principles to gene drives.”
Part of assessing the risks is identifying the concerns of communities and stakeholders about the technology. “There have been regional problem formulation workshops that we, in collaboration with other partners, have supported in different regions of Africa. You do see a lot of commonality. People are interested in potential impacts on human health. Does this genetic modification alter the mosquito’s ability to transmit malaria or other pathogens? You have human health questions like allergenicity. Does the gene produce some protein that causes an allergic reaction? Then you have environmental questions. Do changes to the mosquito have an impact on the ecosystem, particularly valued biodiversity or ecosystem services? Then, because mosquito larval stages are in water, you often also have water quality questions. These are the kinds of concerns that come up consistently across different stakeholder groups.
“Once you identify the concerns, you can assess how likely these are to occur and how much harm they’d cause, and then look at how might you be able to mitigate it. Certainly, one thing which can mitigate the risk is using conventional mosquito control tools that can eliminate mosquito populations if they're applied with sufficient intensity. There aren’t the resources to do that everywhere, otherwise we would eradicate malaria. But if you needed it for a circumscribed area, you could do it.”
Mike says that the areas where the community engagement is most intensive is where the research has actually been taking place. “In those communities, where there are already well established and deep relationship between researchers and the communities, there’s a lot of support for gene drives. But one of the features of gene drives is needing to engage beyond the locations of release, because if the technology works – which is still an if – then you would expect persistence and spread.”
I ask how decisions would be made in Africa, where there are more than 50 countries, most with multiple land borders. “There has been a lot of regional discussion, facilitated by the African Union's Development Agency. There are some mechanisms like in West Africa to actually make regionalized biosafety decisions. There are efforts going on in East Africa right now to build some of those same capacities. The actual decision-making authority is still mostly at the national level. But they are looking at doing that in a way that is transparent and coordinated with neighbouring countries.”
What does good decision-making for technologies such as gene drives look like? This is a question I find particularly informative, so I put it to Mike. “It requires appropriate and relevant information in a form that is clear and understandable. You need the right laws and policies and regulations in place – countries need to be in a position to make a decision, and right now different countries are in different stages of maturity. Then you need the operational processes, things like a mechanism for convening people. You need the right capacity, not just within the regulatory authority, but in the process overall, for example when regulators call in external experts to support them in decision making. You also need processes that support public and other stakeholder consultation. The processes need to be set up to pull the right people in, and then the people that you're pulling in need to feel well prepared.
“It’s important in the context of malaria control to ask what the alternatives are too. There's the consequence of the lack of malaria control, which is death and morbidity and tremendous impact on economies and societies. And other control methods also have pros and cons too. Risk is not reducible to zero in any decision that you make. But regardless of the actual decision that comes out, this is what a good decision looks like. It’s the kind of decision-making that we're trying to support.”
The Sahel is a semi-arid region of Africa, running from Senegal through to Sudan, also including parts of Mauritania, Mali, Burkina Faso, Niger, Chad and northern Nigeria