The Douglas fir dilemma
Can gene technology solve one of our most difficult invasive species decisions (13 minute read)
In the middle of winter, around 25 years ago, I took two staff from the National Parks Service in American Samoa into the Marlborough mountains. They had a week’s gap between the two weeks of a training course they were attending. I helped teach the course, as part of my role with the Department of Conservation, and I was asked if I could show them some of the invasive weed control work going on in New Zealand.
At the time, I was testing out some methods for controlling wild pines in difficult conditions. I was planning a couple of days work in the Leatham Conservation Area, a rugged area of forest, shrubland and alpine tops south-east of Blenheim. It was remote but accessible by four-wheel drive when the Leatham River wasn’t too high. The hut up there had plenty of space, so I arranged for them to come along.
I think it’s fair to say it was a memorable trip for both of them. One had lived all of his life in Samoa, apart from when he’d been to university in Hawai’i. A week-long trip he had once taken to Los Angeles was the coldest he had ever been before I took him up a mountain where there was snow on the ground. The other was originally from Oregon, and couldn’t believe he’d travelled halfway around the world to a place which looked like home.
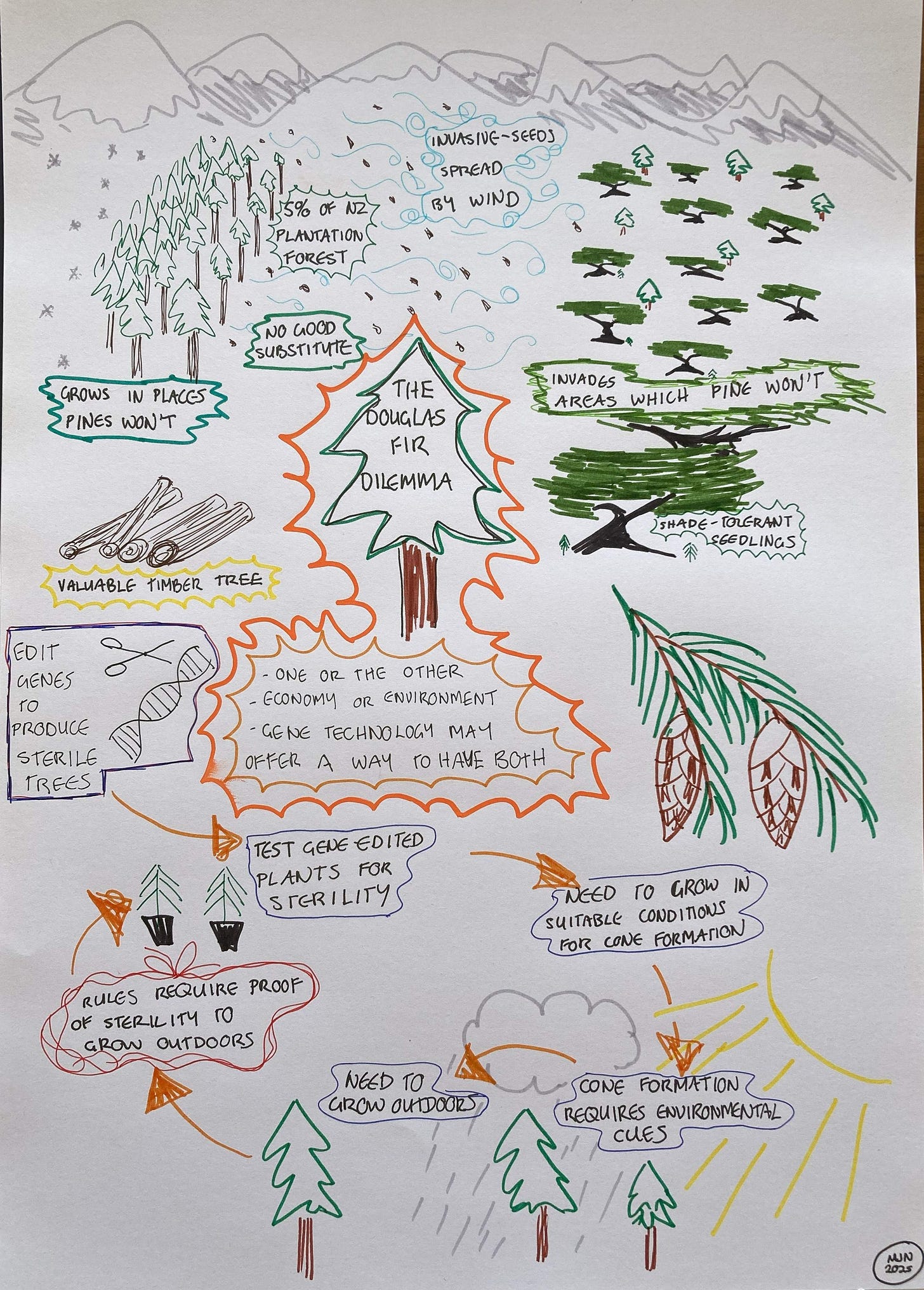
The area chosen for my trials was dominated by wilding pines, along with a tree related to pines called Douglas fir. These trees are known as conifers, because they have cones. New Zealand also has native conifers such as kauri, although our native conifers aren’t closely related to pines and Douglas fir.
From what my colleagues had told me, in the 1950s the Leatham area was considered to have a severe erosion problem. Partly, this was caused by large numbers of introduced mammals like deer and goats. Partly, this was the natural state of the mountains, which have always had large areas of scree – shifting slopes of loose rock inhabited by a delightful collection of tiny plants. At the time, the New Zealand Forest Service, which was managing the land, saw this as a problem to be solved with exotic trees. I actually worked with people who were involved with scattering the seeds of pines and Douglas fir from helicopters over those mountains. The trees grew and spread to become what was considered an intractable problem by the early 2000s. The area affected was so large that the best we could do with our limited funds was try and stop the trees spreading into less invaded areas.
Douglas fir was among the most prominent wild trees in the area, and they intrigued the conservation worker from Oregon. Where I come from, he told us, it’s Douglas fir or it’s a weed. He asked for us to take his picture cutting down and poisoning Douglas firs. Otherwise nobody will believe I was in New Zealand not Oregon, he said. He spent most of the trip exclaiming how much the area looked like home and how amazed he was to find we considered Douglas fir a weed.
It's actually more complicated than that, though. Douglas fir may be our most problematic invasive tree. There are species which are more invasive, but they aren’t planted any more. Douglas fir, on the other hand, is second only to radiata pine in terms of the area planted (although it still makes up only 5% of our exotic forest area). It’s valuable because it makes a good structural timber for houses, and it grows well in cold areas which are less suitable for growing radiata pine. Unfortunately, it’s also tolerant of shade, which means it can cause problems in native forest areas not troubled by light-demanding pines.
When the government announced a review of the gene technology laws, gene editing Douglas fir to be sterile was quoted as an example of what the review might allow. I wanted to know more, because sterile Douglas fir trees would make a huge difference in years to come. We could continue to grow this valuable tree without it invading our forests and mountains.
To learn more, I contacted Scion Research and ended up speaking with Glenn Thorlby, who is a senior scientist in plant biotechnology.
Glenn didn’t start out working on forestry trees. Before he moved to New Zealand in 2009, most of his research was done on a small weed from the cabbage family known as Thale cress or Arabidopsis. At first glance, it doesn’t seem particularly interesting, but it has a relatively small number of genes and a short life cycle. It has become the laboratory workhorse for plant scientists in the same way as fruit flies have for zoologists and E. coli has for many different branches of biology.
“It was very easy to get results,” Glenn tells me. “You get a lot of information in a short time, but there’s no direct benefit.” Working with forestry trees like pine and Douglas fir was a chance to do something which would actually make a difference. But the work brought some challenges.
“The biggest challenge with conifers is that nobody's done that basic stuff. They're very different to some of these model systems [such as Arabidopsis]. In all of our projects we rely on what people have done in other species. But globally there's not much funding for conifers.”
Before gene editing even becomes a possibility, plants have to be grown in what’s known as tissue culture. It’s a time-honoured technique, developed more than 100 years ago, where a fragment of tissue is grown under artificial conditions. Getting it to grow under those conditions is the first, but smaller, challenge. The bigger challenge is taking that tissue and inducing it to form whole plants again.
Conifers are particularly finicky. You can’t use any old material. Glenn says: “in many plants you can pick branches off to proliferate them, but in conifers, if you take a cutting from an old tree it behaves like the old tree and grows like an old tree would grow and doesn't root very well. So you can only use very young material.”
The material Glenn and his team use comes from immature seeds. “We have to collect them from an immature cone and you can only do that for about 5 days in the middle of the Christmas holidays. So for the tissue culture team it's not a lot of fun. They have to test them over those 5 days to pick the time when they're right then cut out the immature embryo from the seed. They have to test those to see if they proliferate and that's probably 20 or 30% of the ones you test. They then have to test to see if they'll also produce embryos which can grow to produce new plants, and that’s a smaller percentage again. It's a lot of work.”
If it does work, though, the technique can produce a huge number of plants. Material can be preserved by freezing and then thawed out, but this can’t be done too many times. “You can’t take them out of the cryopreservation and grow them and put them back in – once you've done that a couple of times, they're never quite as good.”
All of this work only gets you to the start line, though. For the next stage, Glenn and his team had to figure out which genes to look at. He explains: “we selected some genes which were the standard genes for reproductive development in species like Arabidopsis. Then, because there isn't much known about conifers, we selected a couple of conifer genes which people have suggested might be involved in cone development. In that project we carried out editing of, I think, 4 genes that we selected, so that they should be non-functional. And so in theory these may be sterile. This has been pretty successful.”
But Glenn and his team had reached this point before. They were the first in the world to produce a gene edited conifer tree – as you’d expect, radiata pine – which they grew in their approved containment site. It’s the only field trial in New Zealand for any kind of genetically modified plant. However, there are strict containment conditions, Glenn explains. “You're not allowed to let what they call heritable material leave, which means pollen and seed. And so the conditions of our radiata trial is that we have to kill the trees before they reproduce. And so we have to inspect every branch every 2 weeks for much of the year, and if we find any sign of transitioning to reproduction, we have to kill that plant within 7 days and cut off the reproductive buds and autoclave1 them.”
The problem with testing the Douglas fir, however, is that they need to allow it to at least begin developing cones, so they can figure out whether or not the trees are sterile. “We approached the EPA for discussions about how we might progress with this. You have to grow Douglas fir outside, because it takes 12 years to produce cones, they have to get big, and they need environmental cues. The EPA suggested they wanted proof that the trees were sterile before we could have that sort of trial to grow Douglas fir outside. We went back and forwards for a year and then we decided not to pursue it, because we couldn’t provide the proof they wanted unless we had a field trial. So at that point we decided to freeze the material to preserve it. We have been exploring with Oregon State University whether under the more permissive US regulations they could test the trees for us, but the US regulator has not made a decision about this yet. So when the government announced these new regulations, I guess that spurred a lot of interest.”
Some of what I’ve read from government announcements suggested that the gene-edited Douglas fir trees might be entirely deregulated, but Glenn suggests it’s a bit more complex than that, because of the way the trees have been developed. “These are transgenic trees, which also have an edit.” Glenn explains that this means that there has been some new DNA added to the trees, as well as edits to the DNA which may affect the ability to reproduce. This is a common technique in gene editing plants, because it makes them easier to produce. However, with many crops they then use traditional cross-breeding to produce plants which have the edit but not the new DNA. “But obviously we can't do that because we have edited the plants for sterility.”
Nonetheless, Glenn does consider the trees his team are producing to be a low risk. “The perceived risk is that the genes you put in can spread. If the plants are sterile, that's not such a risk. That's one of the main things people want to know. When we go to public forums, people want to know whether the plants will cross with kauri. But these conifers don’t cross with natives. That's not a biological possibility.”
To put this in perspective, plants are classified by botanists according to their family trees. The conifers Glenn and his team work on belong to a different branch of that family tree from New Zealand’s conifers. These groups don’t cross with each other. Some genetically modified crops have crossed with non-modified plants, but these crosses have been expected crosses, such as between cultivated and wild soybean, not cases where completely unrelated plants have begun crossing.
Another factor which puts the sterile Douglas fir into a lower risk category is that the kinds of edits Glenn and his team are doing are mostly no different from what could occur naturally by mutation and evolution or could be produced from conventional breeding. The trait they are developing, too, is something which could easily occur in nature. He points out that there are probably sterile Douglas fir growing somewhere, if only you could find them. But even if they found one, they couldn’t propagate it, he points out, because if you propagate an old tree, you just get an old tree.
It's certainly the case that there are numerous examples of sterile plants which occur naturally or are produced by conventional breeding. It’s not like using genes from jellyfish or coral to create glow-in-the-dark fish, for example, or using genes from bacteria to make plants resistant to insects. However, we shouldn’t place too much weight on the perceived naturalness of gene-edited or modified plants. Traits which result from evolution or conventional breeding are not intrinsically safer than those developed with gene technology. After all, many plants naturally contain lethal toxins. On the whole, we are inclined to worry less about risks we see as natural rather than industrial or artificial, but that doesn’t mean they won’t harm us.
In fact, this is consistent with Glenn’s thoughts on the regulation of gene technology. He describes the current laws in New Zealand as “process-driven, whereby, just by carrying out the manipulation it's regulated.” He’d prefer to see something “product based, which is kind of what the government's proposing. It’s what's produced at the end which will be assessed”.
I asked Glenn directly for his views on the risks of gene technology in general, because I wanted to understand how someone who works in the field might see it. I wanted to know whether anything really worried him. “It’s likely people are working on biological weapons or things which I'm sure anybody is worried about. I think, like with any technology, there could be evil users.”
I also wondered about unintended consequences – was this something he’d seen with gene technology. He explained: "it depends what you mean by unintended consequences. Because if you’re using it as a research tool, you’ll obviously get a lot of what you might call unintended consequences. We did some work modifying lignin [the complex molecule which gives wood its structure] to make trees more suitable for use as a biofuel. But lignin is important in supporting the tree and transporting water so the modified tree doesn’t grow well, and it's a dwarf. So I mean, they are kind of unintended consequences. This is why the products of genetic technologies undergo comprehensive testing before they go to market.”
But, he points out, this does happen with conventional breeding too. “There’s a famous case where they crossed potatoes to create a new variety (Lenape) with improved insect resistance that was released for commercial production. They were resistant because they had a lot of glycoalkaloids which are also human toxins and following reports of illness from eating them they were removed from sale. This led to improved testing of the products of conventional breeding.”
That’s why, he points out, you have to test things. And this comes back to the issue with Douglas fir. There’s material which might grow sterile trees, but we don’t know, because the current rules make testing almost impossible. Here, I go back to the importance of considering risks in context. The attempts of Glenn and his team to produce sterile trees isn’t happening in a vacuum. We currently have an important forestry tree which is also a serious invasive species. An invasive forestry tree isn’t like an invasive garden plant where there’s usually a less invasive substitute. For Douglas fir, Glenn tells me: “there's a supply chain. There are mills working on it. The alternatives aren’t as good. But they aren’t planting much right now because of the problem of wild Douglas fir.”
This leaves us with a choice. Without gene technology, it’s a zero-sum game between the economy or the environment. We either continue to plant an invasive tree because it’s valuable and important, or we sacrifice a valuable tree because it harms the environment. Gene technology offers a third option, potentially one where we could have the benefits of Douglas fir without the environmental harm. It’s a matter of opinion, and personal values, which of the three options is preferable. However, this is the kind of situation where my own opinion leans towards gene technology as the best choice.
An autoclave unit uses high temperatures and pressure to sterilise material.






Ever since I was a child, I've loved Douglas fir trees. Native to where I live, it takes me a little getting used to the idea that they are invasive elsewhere.
How very interesting.